Delcath Systems, Inc. Announces Promising Results from Independent Study on Repeated Hepatic Chemosaturation for Liver Tumors
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240826877468/en/
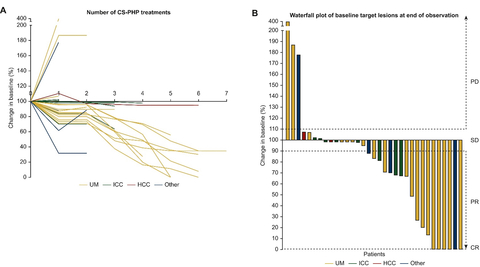
Figure 1: Individual changes in tumor sizes during CHEMOSAT treatment. (A) Individual changes in tumor sizes during CHEMOSAT treatment. On the y-axis the change in tumor size is indicated according to the baseline lesions. (B) Waterfall plot of changes in the size of target lesions at the end of observation. Dashed lines are thresholds for hepatic PD, hepatic SD, hepatic PR and hepatic CR. CR, complete response; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocellular carcinoma; other, ciliary body melanoma, angiosarcoma, tonsil and pancreatic carcinoma; PD, progressive disease; PR, partial response; SD, stable disease; UM, uveal melanoma. (Graphic: Business Wire)
Key Findings from the Independent Study:
- Patient Population: This retrospective study evaluated the efficacy of CHEMOSAT in 33 previously treated patients with unresectable intrahepatic metastases from various cancers: uveal melanoma (N=19), cholangiocarcinoma (N=8), hepatocellular carcinoma (N=2), and one patient each with ciliary body melanoma, acinar cell carcinoma, pancreatic cancer, or tonsil cancer (N=4). In addition to hepatic metastases, 7 out of 33 patients also had limited extrahepatic disease, which was found not to significantly impact overall survival.
- Disease Control Rate: The study reported a disease control rate (DCR) of 91%, with 30 out of 33 patients experiencing either objective tumor response or stable disease. Notably, 6 patients (18.2%) achieved complete response (CR) in the liver, including 5 patients with uveal melanoma and 1 patient with cholangiocarcinoma, who received a median of 5 treatment cycles.
- Hepatic Progression-Free Survival: Median hepatic progression-free survival (hPFS) was 52 weeks across all patients, with particularly strong outcomes for specific cancers:
- 69 weeks (16 months) median hPFS in patients with uveal melanoma.
- 38 weeks (8.5 months) median hPFS in patients with cholangiocarcinoma.
- Importance of Repeated Treatments: The investigators’ approach of using CHEMOSAT in the form of regularly repeated treatment cycles as clinically indicated (Figure 1), similar to systemic chemotherapy, resulted in long-term disease control in the majority of patients and was well tolerated.
- Tolerability and Safety: The safety profile of CHEMOSAT was consistent with published literature. Most patients experienced transient hematological adverse events, which were routinely managed with supportive care. Importantly, no significant liver damage was reported, even in patients who underwent multiple treatment cycles. Treatment was discontinued in 2 patients due to adverse events, and 2 patients withdrew consent during the treatment period.
Dr.
The publication is available here.
About
In
In
View source version on businesswire.com: https://www.businesswire.com/news/home/20240826877468/en/
Investor Relations:
ICR Westwicke
investorrelations@delcath.com
Source:
